Experience
1. Studies & Services
1-1. Summary
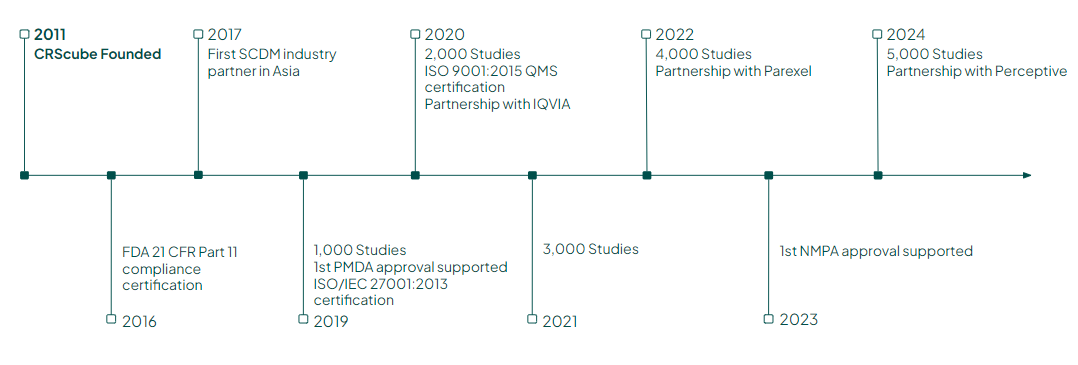 | |||
Studies | Site Users | Patients | Regulatory Approvals |
+6,300 | +91,000 | +1,980,000 | +350 |
1-2. #No of Studies per Year and Phase
YEAR | P1 | P2 | P3 | P4 | IIT | MD | PMS | OS | BE | FS | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
2019 | 44 | 29 | 33 | 13 | 11 | 29 | 29 | 21 | 266 | 25 | 3 | 503 |
2020 | 74 | 67 | 37 | 21 | 41 | 35 | 49 | 40 | 317 | 61 | 11 | 753 |
2021 | 75 | 46 | 42 | 13 | 36 | 42 | 53 | 39 | 492 | 47 | 7 | 892 |
2022 | 88 | 32 | 40 | 17 | 38 | 50 | 55 | 33 | 347 | 65 | 14 | 779 |
2023 | 114 | 33 | 48 | 10 | 40 | 85 | 68 | 32 | 257 | 60 | 13 | 760 |
2024 | 176 | 43 | 61 | 17 | 38 | 74 | 71 | 42 | 238 | 75 | 16 | 851 |
2025 | 161 | 23 | 32 | 21 | 43 | 66 | 69 | 48 | 238 | 35 | 15 | 751 |
Total (Since 2010) | 769 | 384 | 434 | 239 | 277 | 385 | 537 | 373 | 2297 | 355 | 74 | 6340 |
1-3. #No of Studies per Therapeutic Area and Phase
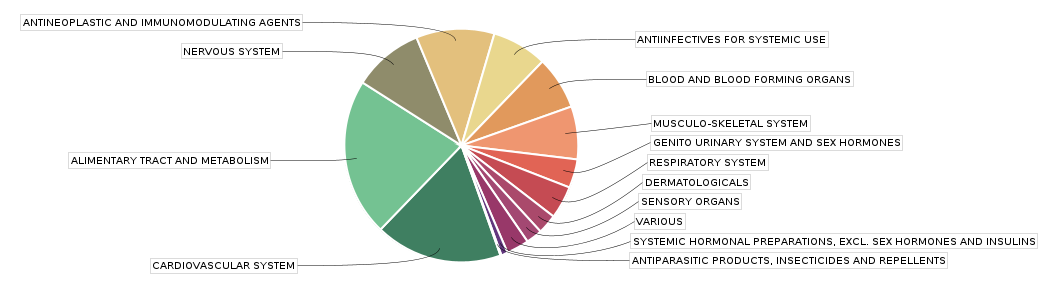
Therapeutic Areas | P1 | P2 | P3 | P4 | MD | IIT | PMS | OS | BE | FS | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
ALIMENTARY TRACT AND METABOLISM | 41 | 28 | 64 | 36 | 0 | 23 | 53 | 32 | 388 | 102 | 110 | 775 |
CARDIOVASCULAR SYSTEM | 28 | 8 | 61 | 36 | 0 | 17 | 67 | 74 | 324 | 4 | 4 | 619 |
ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | 71 | 80 | 14 | 25 | 0 | 55 | 83 | 28 | 16 | 5 | 15 | 387 |
NERVOUS SYSTEM | 8 | 10 | 13 | 28 | 2 | 11 | 30 | 19 | 208 | 4 | 8 | 335 |
ANTIINFECTIVES FOR SYSTEMIC USE | 24 | 33 | 29 | 16 | 0 | 5 | 47 | 13 | 94 | 0 | 7 | 268 |
BLOOD AND BLOOD FORMING ORGANS | 14 | 31 | 14 | 9 | 1 | 18 | 15 | 29 | 113 | 0 | 6 | 249 |
MUSCULO-SKELETAL SYSTEM | 16 | 27 | 23 | 15 | 4 | 7 | 21 | 8 | 116 | 6 | 13 | 246 |
RESPIRATORY SYSTEM | 3 | 7 | 18 | 4 | 0 | 3 | 26 | 9 | 83 | 3 | 4 | 157 |
GENITO URINARY SYSTEM AND SEX HORMONES | 7 | 3 | 10 | 3 | 0 | 5 | 11 | 10 | 84 | 8 | 8 | 141 |
VARIOUS | 10 | 7 | 3 | 6 | 29 | 9 | 8 | 11 | 4 | 10 | 46 | 104 |
DERMATOLOGICALS | 8 | 13 | 17 | 2 | 11 | 7 | 11 | 3 | 10 | 10 | 22 | 93 |
SENSORY ORGANS | 11 | 16 | 18 | 9 | 0 | 2 | 12 | 7 | 1 | 2 | 4 | 80 |
SYSTEMIC HORMONAL PREPARATIONS, EXCL. SEX HORMONES AND INSULINS | 1 | 1 | 3 | 4 | 0 | 3 | 6 | 6 | 7 | 0 | 0 | 31 |
ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
[UNKNOWN] | 217 | 47 | 33 | 12 | 211 | 43 | 22 | 32 | 463 | 106 | 342 | 1211 |
NOT DEFINED | 7 | 9 | 29 | 0 | 7 | 2 | 2 | 4 | 8 | 2 | 50 | 111 |
1-4. #No of Services per Year
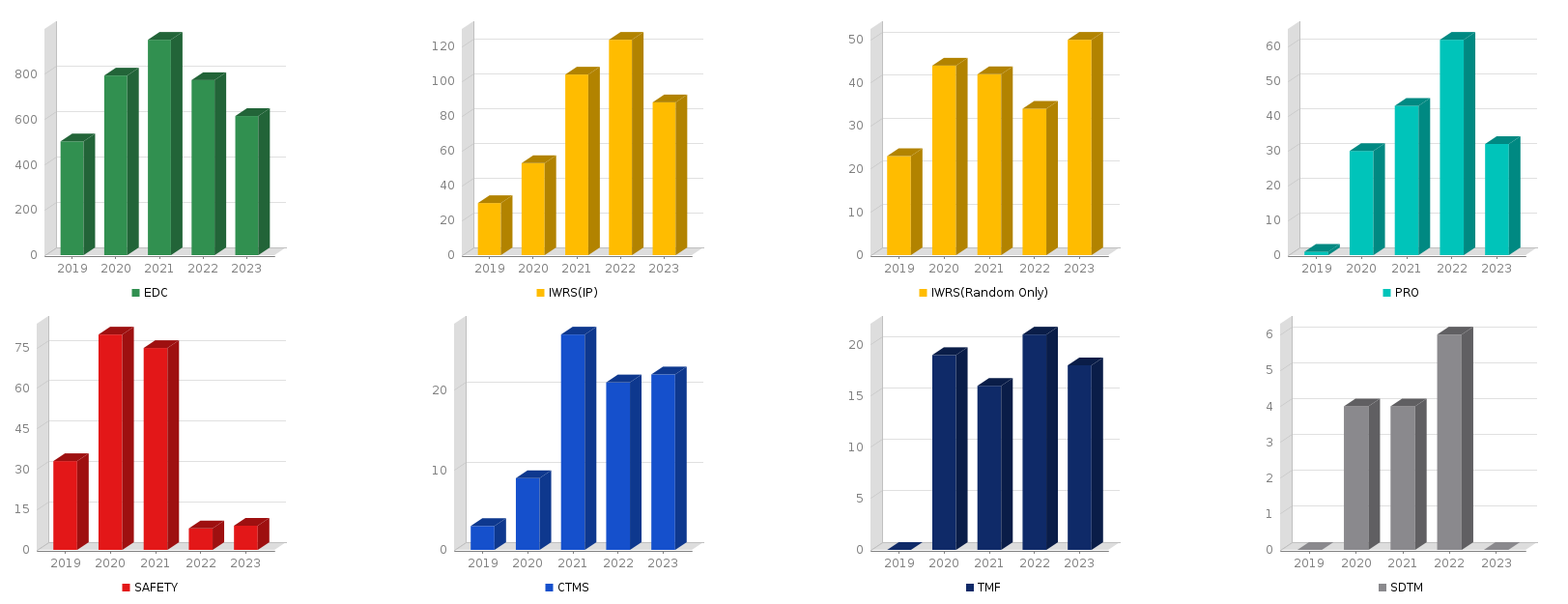
YEAR | EDC | IWRS (IP) | IWRS (Random Only) | PRO | cubeSAFETY | CTMS | TMF | DM | SDTM |
|---|---|---|---|---|---|---|---|---|---|
2019 | 503 | 30 | 23 | 1 | 41 | 3 | . | 17 | . |
2020 | 749 | 50 | 40 | 30 | 80 | 9 | 19 | 31 | 4 |
2021 | 891 | 96 | 42 | 40 | 77 | 30 | 16 | 31 | 4 |
2022 | 774 | 123 | 35 | 62 | 9 | 17 | 17 | 20 | 6 |
2023 | 736 | 112 | 58 | 46 | 12 | 29 | 25 | 18 | . |
2024 | 842 | 137 | 56 | 49 | 15 | 23 | 17 | 9 | 1 |
Total | 4516 | 551 | 255 | 232 | 234 | 112 | 98 | 126 | 15 |
1-5. #No of DM Studies per Year
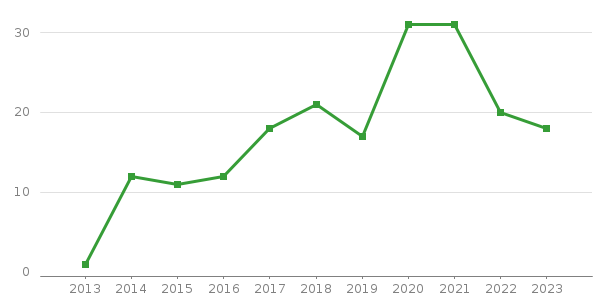
Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
Total | 1 | 12 | 11 | 12 | 18 | 21 | 17 | 31 | 31 | 20 | 18 | 9 |
2. Global Experience
2-1. US
Sponsor | Phase | Indication | Note |
|---|---|---|---|
P** (Biotech) | II | Oncology | |
H** (Biotech) | I/II | LARS (Low Anterior Resection Syndrome) | |
O** (Biotech) | II | COVID 19 | Emergency Use Authorization expected in the 1st quarter of 2024 cubeCDMS + cubeSAFETY |
V** (Biotech) | I | Oncology | |
V** (Biotech) | II | Oncology | cubeCDMS + cubeSAFETY |
O** (Biotech) | II | Atopic Dermatitis | |
N** (Biotech) | I | Oncology | cubeCDMS + cubeIWRS |
2-2. EU
Sponsor | Phase | Indication | Note |
|---|---|---|---|
S** (Pharmaceutical company) | OS | NA | Bulgaria, Czechia, France, Poland, Romania, Russia, Ukraine |
M** (Biotech) | III | NA | Russia |
D** (Biotech) | Pivotal | Transrectal Prostate Biopsy | Poland |
S** (Biotech) | Pivotal | Prediabetes | Hungary, Romania, Slovakia |
G** (Pharmaceutical company) | III | NA | Poland |
P** (Biotech) | II | Diabetes | Poland |
P** (Pharmaceutical company) | I | NA | Poland |
P** (Pharmaceutical company) | I&II | Primary Progressive Multiple Sclerosis, PPMS | Poland |
S**(Pharmaceutical company) | II | Ophthalmic/ocular Diseases | Poland |
S**(Pharmaceutical company) | III | PGTCS | Serbia, Macedonia |
C**(Pharmaceutical company) | III | Rheumatoid Arthritis | Poland |
C**(Pharmaceutical company) | III | Plaque Psoriasis | Poland |
N**(Pharmaceutical company) | OS | NA | Poland |
2-3. Phase 3
Country | Regulation Agency | No of Studies |
|---|---|---|
Korea | MFDS | More than 30 per year |
Japan | PMDA | 36 |
China | NMPA | 12 |
Taiwan | TFDA | 2 |
EU | EMA | 6 |
2-4. Regulatory Body Inspection
In most regulatory authorities, conducting inspections solely for eClinical solutions is rare. Still, we have compiled the following summary in response to commonly asked questions from our clients. The parentheses next to the number of studies indicate the following categories:
Some eClinical solutions were included in the study approval inspection process.
Inspection on eClinical solutions.
Country | Regulatory Agency | No. of Studies | Notes |
|---|---|---|---|
Korea | MFDS | >= 15 per year (1) | |
Japan | PMDA | 10 (1) | |
China | NMPA | 1 (1) 3 (2) | (1) Small cell lung cancer. (2) NMPA inspection on eClinical solutions used in China. |
Rwanda | Rwanda FDA | 1 (2) | COVID-19 vaccine. |
Philippines | Philippine FDA | 1 (2) | COVID-19 vaccine. |
USA | FDA |
|
|
Taiwan | TFDA | NA | |
EU | EMA | NA |
2-5. Studies per Region and Phase
Region | P1 | P2 | P3 | P4 | MD | IIT | MS | OS | BE | FS | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Korea | 354 | 229 | 278 | 202 | 248 | 124 | 375 | 270 | 1857 | 259 | 572 | 4261 |
Japan | 51 | 45 | 28 | 3 | 12 | 82 | 36 | 15 | 22 | 2 | 21 | 303 |
China | 48 | 30 | 11 | 0 | 0 | 2 | 2 | 4 | 38 | 1 | 2 | 137 |
Taiwan | 1 | 2 | 0 | 0 | 1 | 0 | 1 | 4 | 0 | 0 | 1 | 9 |
US | 1 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 6 |
Europe | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 7 | 0 | 0 | 1 | 10 |
ROW | 9 | 10 | 16 | 2 | 0 | 1 | 1 | 14 | 0 | 0 | 0 | 53 |
