Qualifications
GAMP 5 Compliance
GAMP 5 (Good Automated Manufacturing Practice 5) is a risk-based framework for validating computerized systems in the pharmaceutical and healthcare industries, ensuring compliance with regulatory requirements such as those from the FDA and EMA.
Assessed and qualified by an independent computer system validation service provider (Zifo).
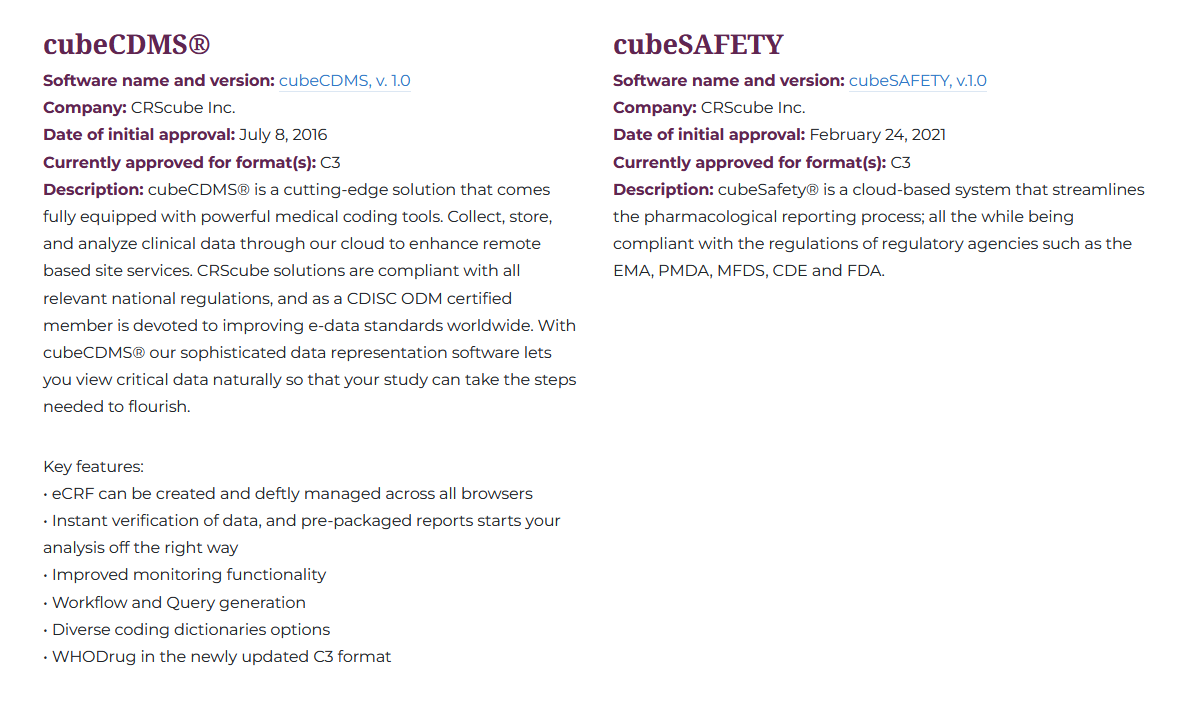
1. cubeCDMS

2. cubeSAFETY

ISO/IEC 27001:2022
ISO/IEC 27001:2022 is the latest version of the international standard for information security management systems (ISMS), providing a framework for establishing, implementing, maintaining, and continually improving information security to protect organizations from risks such as cyber threats, data breaches, and regulatory non-compliance.
Re-issue Date:

ISO 9001:2015
ISO 9001:2015 is an international standard for quality management systems (QMS) that provides a framework for organizations to consistently meet customer and regulatory requirements while enhancing process efficiency and continuous improvement.
Initial Issue Date:
Certificate Issue Date:
Expiration Date:

WHODrug Software Certification
Uppsala Monitoring Centre certifies software systems that are approved within the WHODrug Vendor Programme for handling B3/C3 format data.
Source: https://who-umc.org/whodrug/whodrug-vendor-programme/approved-software-systems/
